Recognising that clinical trials can be demanding for patients, the opportunity to participate fully or partially from home could shift some burdens away from patients and also extend participation to a wider patient population.
An earlier study carried out across our British community and other European countries found that 57% of patients preferred participating mostly from home and 19% preferred participating fully from home.
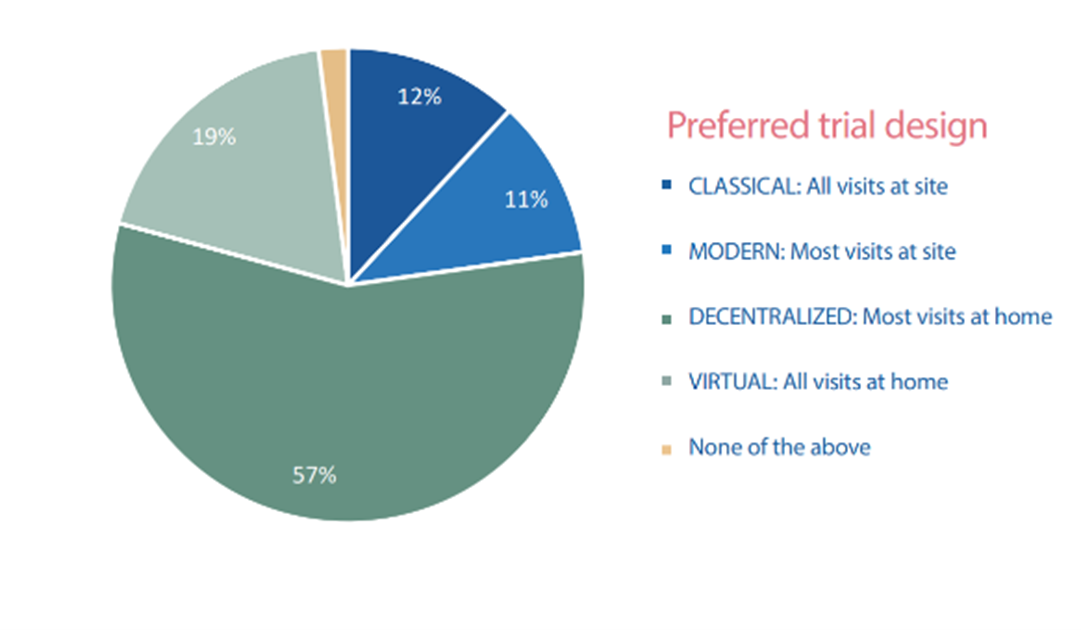
Only 12% of people preferred the classical trial design with all visits being at the hospital!
So, what are the reasons for these preferences and how do they have the potential to ease clinical trial participation?
Increased Flexibility
“I would like to participate from home as it would mean that I wouldn’t have to travel. Things could be done over the phone or online, so I wouldn’t have to exert so much energy to take part which would reduce the burdens. I wouldn’t have any concerns participating from home,” says Carol who is living with COPD.

As part of our research to understand the clinical trial participation experience of those with lung conditions, we received 943 responses to our questionnaire and also conducted numerous interviews.
Head Nurse, Birgit Hauggaard Nielsen added:
“Sometimes people with severe lung diseases can be stressed at the thought of traveling to a hospital. The ability to do home visits as a part of the clinical study design could be a positive alternative for people with severe lung diseases.”
Many of the demands of clinical trials become the responsibility of the patient, yet it is patients that tend to face more challenges and be less able than those running the trial. The opportunity to participate from home lifts some responsibilities from patients and with sufficient guidance, they are still able to feel supported.
Guidance and Communication
Although participating partially or fully from home is the preference, some worries such as technical difficulties and managing wearable devices are present.
This is where guidance and communication with nurses is essential. Patients must feel they are receiving sufficient education to monitor things from home and know that they have a contact point if needed.

“There are many possibilities in terms of collecting data from participants in a clinical trial. It’s important that patients feel comfortable and safe using the technology and know that they have a responsible person to call if they need help,” explains Birgit.
Monthly retention calls, sufficient education and guidance as well as a direct line to nurses would all ease the worries of participating from home.
Some patients who feel more competent using technology such as Cliff expressed having no concerns: “I like the idea of being able to participate from home, because it would ease travel time and burdens. I wouldn’t have any concerns participating from home”.
Including More Patients
Lifting the demands of travel and stressful journeys from patients would enable a wider patient population to participate and would therefore enable trials to be more inclusive.
“Offering participation in clinical trials with home visits allows all patients the opportunity to participate if they are interested. Without this option, participating with every visit in person can prevent some eligible patients from participating,” explains Birgit.
Birgit then added:
It’s about giving every patient the opportunity to get to know about engaging in clinical trials, not just those who have the resources and the ability to travel to the sites.
The willingness to participate is evident and the option to participate from home would not only reduce the burdens for patients, but also be more inclusive and accommodating for patients.
Ensuring that patients continue to feel like a priority and that their needs are heard and valued is what we constantly strive for at the British Research Panel! Share your thoughts on participating from home in the comments below!
We want to thank all of those who continue to be an active part of our community and participate in trials, interviews and surveys.
You are welcome to take a look at our previous report on participating from home: The-Patients-Perspective-on-Decentralized-Trials-2.pdf (jameslindcare.com)
You can also hear about one of our members who participated in a webinar to share her personal experiences regarding participating in a clinical trial with a lung condition: Click here
Written by
Eloise Healey

